A method to calculate new valence electronegativity and the ionicity of chemical bond of the transition metal elements is also presented here.
提出了计算过渡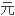
 价态共价半径的公式;提出了计算过渡
价态共价半径的公式;提出了计算过渡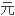

 价态电负性和化学键的离子性的方法。
价态电负性和化学键的离子性的方法。
A method to calculate new valence electronegativity and the ionicity of chemical bond of the transition metal elements is also presented here.
提出了计算过渡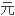
 价态共价半径的公式;提出了计算过渡
价态共价半径的公式;提出了计算过渡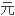

 价态电负性和化学键的离子性的方法。
价态电负性和化学键的离子性的方法。
The essential feature of monomer molecules is the ability to form chemical Bonds (see Bonding) with at least two other monomer molecules (polyfunctionality).
单体分子的基本特性是至少能与两

 单体分子形成化学键的能力(多功能性)。
单体分子形成化学键的能力(多功能性)。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核, 表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
A method to calculate new valence electronegativity and the ionicity of chemical bond of the transition metal elements is also presented here.
提出了计算过渡元素价态共价半径的公式;提出了计算过渡元素新价态

 和化学键的离子
和化学键的离子 的方法。
的方法。
The essential feature of monomer molecules is the ability to form chemical Bonds (see Bonding) with at least two other monomer molecules (polyfunctionality).
单体分子的基本特 是
是
 与两个其它单体分子形成化学键的
与两个其它单体分子形成化学键的 力(多功
力(多功
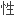 )。
)。
声明:以上例句、词 分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
A method to calculate new valence electronegativity and the ionicity of chemical bond of the transition metal elements is also presented here.
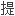
 了计算过渡元素价态共价半径的公
了计算过渡元素价态共价半径的公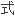 ;
;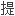
 了计算过渡元素新价态电负性和
了计算过渡元素新价态电负性和
 键的离子性的方法。
键的离子性的方法。
The essential feature of monomer molecules is the ability to form chemical Bonds (see Bonding) with at least two other monomer molecules (polyfunctionality).
单体分子的基本特性是至少能与两个其它单体分子形
 键的能力(多功能性)。
键的能力(多功能性)。
声明:以上例句、词性分类均由互联网资源自动生 ,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
A method to calculate new valence electronegativity and the ionicity of chemical bond of the transition metal elements is also presented here.
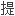
 了计算过渡元素价态共价半径的公式;
了计算过渡元素价态共价半径的公式;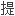
 了计算过渡元素新价态电负
了计算过渡元素新价态电负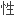 和化学键的离子
和化学键的离子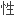 的方法。
的方法。
The essential feature of monomer molecules is the ability to form chemical Bonds (see Bonding) with at least two other monomer molecules (polyfunctionality).
单体 子的基本特
子的基本特 是至少能与两个其它单体
是至少能与两个其它单体 子形成化学键的能力(多功能
子形成化学键的能力(多功能 )。
)。
声明:以上例句、词
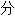
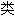 均由互联网资源自动生成,部
均由互联网资源自动生成,部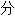 未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
A method to calculate new valence electronegativity and the ionicity of chemical bond of the transition metal elements is also presented here.
提出了计算过渡元素价态共价半径的公式;提出了计算过渡元素新价态电负性和化学键的离子性的方法。
The essential feature of monomer molecules is the ability to form chemical Bonds (see Bonding) with at least two other monomer molecules (polyfunctionality).
单体分子的基本特性是至少能与两个其它单体分子形成化学键的能力(多功能性)。
声明:以上例句、词性分类均由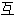
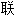
 资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
A method to calculate new valence electronegativity and the ionicity of chemical bond of the transition metal elements is also presented here.
提出了计算过渡元素价态共价半径的公式;提出了计算过渡元素新价态电负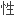 和
和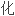

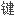 的离子
的离子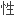 的方法。
的方法。
The essential feature of monomer molecules is the ability to form chemical Bonds (see Bonding) with at least two other monomer molecules (polyfunctionality).
单体分子的基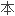

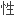 是至少能与两个其它单体分子形成
是至少能与两个其它单体分子形成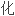

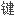 的能力(多功能
的能力(多功能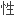 )。
)。
声明:以上例句、词 分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表
分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表 软件的观点;若发现问题,欢迎向我们指正。
软件的观点;若发现问题,欢迎向我们指正。
A method to calculate new valence electronegativity and the ionicity of chemical bond of the transition metal elements is also presented here.
提出了计算过渡元素价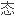 共价半径的公式;提出了计算过渡元素新价
共价半径的公式;提出了计算过渡元素新价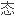

 性和化学键的离子性的方法。
性和化学键的离子性的方法。
The essential feature of monomer molecules is the ability to form chemical Bonds (see Bonding) with at least two other monomer molecules (polyfunctionality).
单体分子的基本特性是至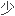

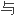 两个其它单体分子形成化学键的
两个其它单体分子形成化学键的 力(多功
力(多功 性)。
性)。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
A method to calculate new valence electronegativity and the ionicity of chemical bond of the transition metal elements is also presented here.
提出了计算过渡元素价态共价半径的公式;提出了计算过渡元素新价态电负性和化学键的离子性的方法。
The essential feature of monomer molecules is the ability to form chemical Bonds (see Bonding) with at least two other monomer molecules (polyfunctionality).
单体分子的基本特性是至少能与两个其它单体分子形成化学键的能力(多功能性)。
声明:以上例句、词性分类均由互

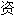 源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
A method to calculate new valence electronegativity and the ionicity of chemical bond of the transition metal elements is also presented here.
提出了计算过渡元素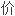
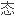
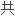
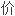 半径的公式;提出了计算过渡元素新
半径的公式;提出了计算过渡元素新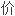
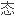 电负性和化学键的离子性的方法。
电负性和化学键的离子性的方法。
The essential feature of monomer molecules is the ability to form chemical Bonds (see Bonding) with at least two other monomer molecules (polyfunctionality).
单体分子的基本特性是至少 与两个其它单体分子形成化学键的
与两个其它单体分子形成化学键的 力(
力(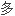
 性)。
性)。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。